Design Controls
Developing any Product requires a sound strategy. In Medical Devices, that starts and ends with Design Controls
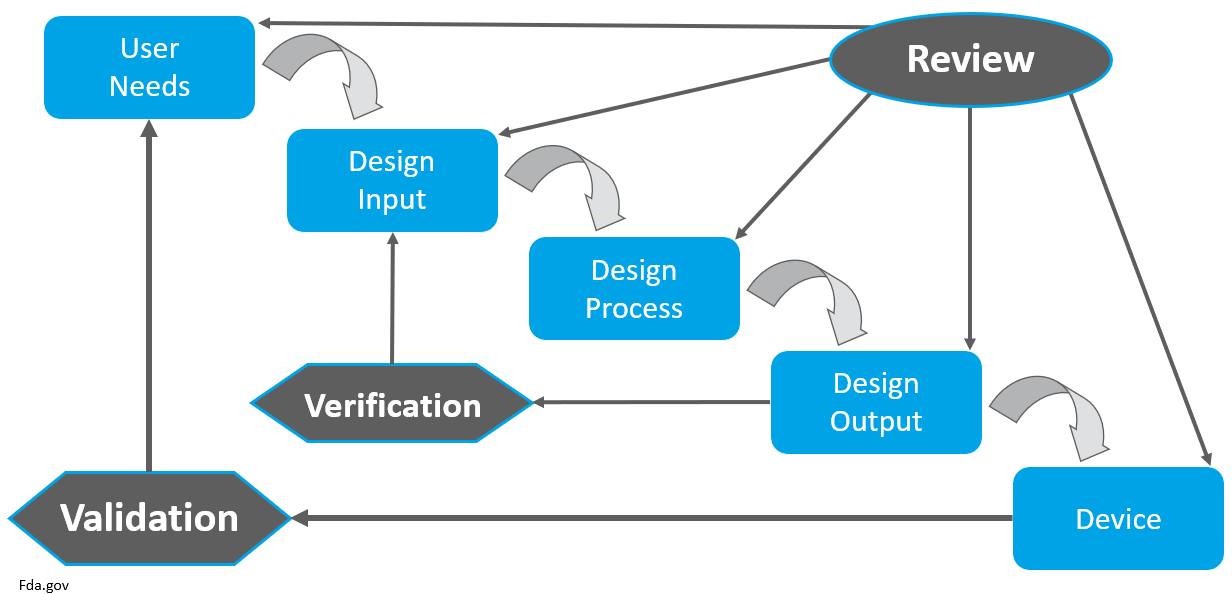
Design controls are key in assuring devices meet user needs, intended uses, and specific requirements. The process can be broken down into design stages. Companies can tailor their own design stages to fit their unique systems. Below is an example of how these design stages can be broken down, and identifies the key activities performed within each stage
-
Market and customer needs
Technical competencies
Manufacturability
Regulatory pathways
Patents
Development costs
Design and Development Plan
-
Design and Development Plan
Design inputs
User requirements
Indications for use
Design outputs
Specifications
Drawings
Labeling
Product codes
IFU/DFU
Surgical techniques
Risk management
Safety and biocompatibility
Initial Design Verification
-
Test design to ensure design outputs meet inteded use
Validate manufacturing processes
Validate final design with users
-
DHF finalized
Design change process controlled and enforced
Market feedback